Stunning Info About How To Spot A Chemical Reaction

A reversible reaction is a simultaneous conversation of reactants into products and products into reactants.
How to spot a chemical reaction. In order to know if you are witnessing a chemical reaction, look for these telltale signs: There has been a colour change inside the reaction flask. Identify the cation and anion of the compounds involved in the reaction, as well as their charges.
So we are good to go for step 1. How to tell if a chemical reaction has occurred? To identify a redox reaction, we must first calculate the oxidation number of each atom in the reaction.
An example of a reversible reaction is h 2 g + i 2 g ⇌ 2 hi. How to tell if a chemical reaction has occurred? What are 10 signs of a chemical reaction?
You shouldn't he spotting directly from a reaction mixture without diluting. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side of the reaction. We can tell if a chemical reaction has taken place when one or more of the following things happen:
On the other hand, water ( {eq}h_2 o {/eq}) and carbon dioxide ( {eq}co_2. It's important to understand that the inte. If there is a change in oxidation number, then the reaction is a redox reaction.
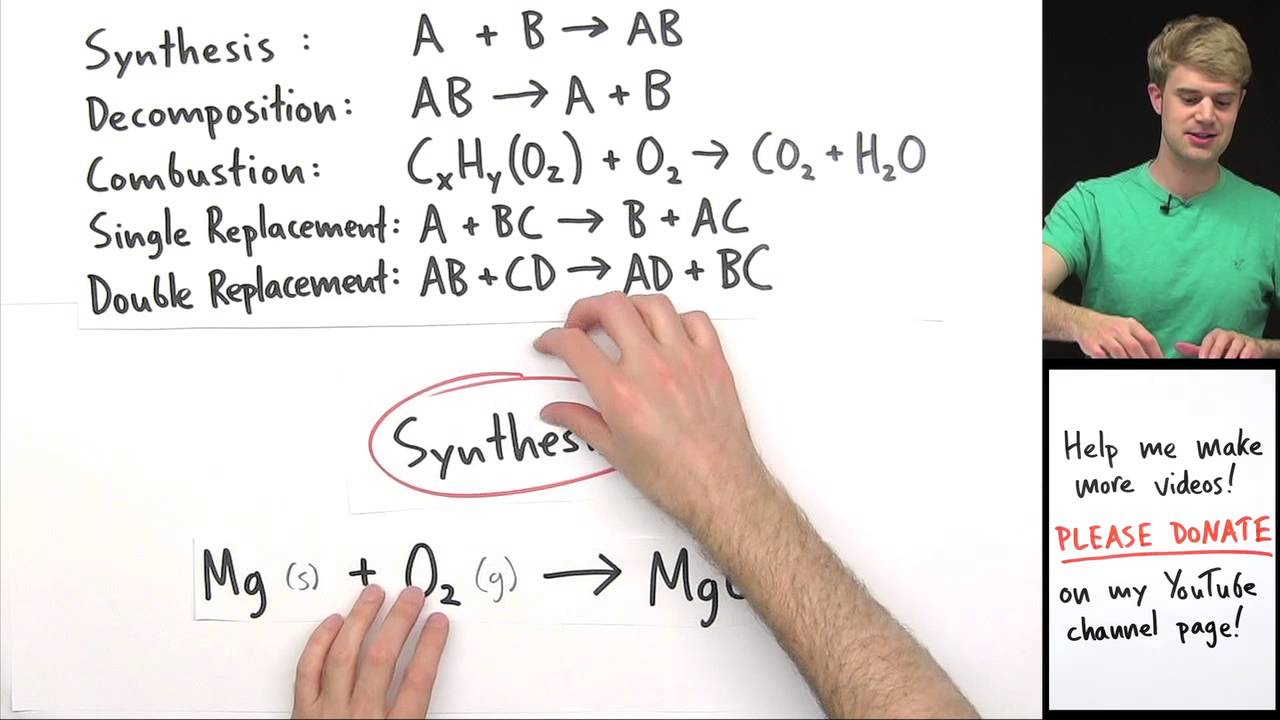
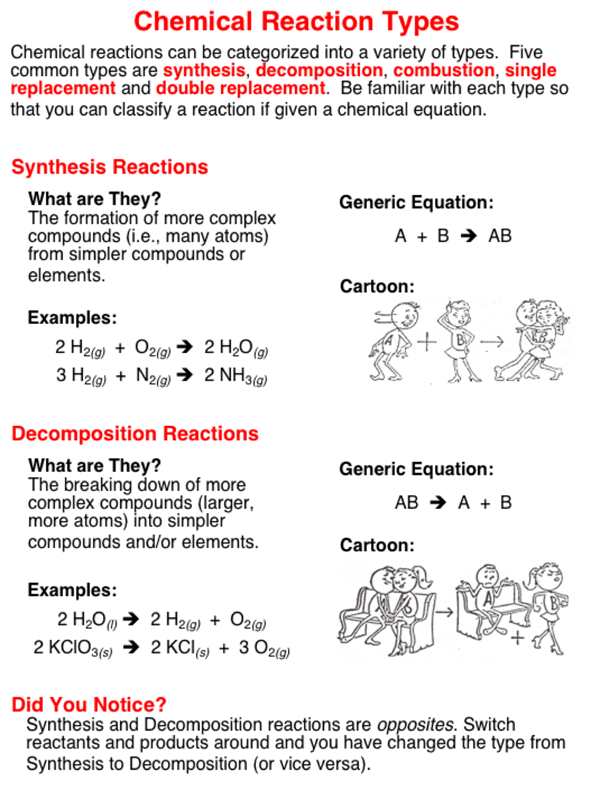
This video looks at how to identify the five reaction types presented in most introductory chemistry texts: Production of gas (bubbling) formation. There are five signs of a chemical change:


/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)